 Research article
Research article
Favoring Factors for Urinary Tract Infections due to Fluoroquinolone-Resistant Escherichia Coli in A Tertiary Care Department – Single Center Experience
Velma Rebic1, Mufida Aljicevic1, Amela Dzubur1, Edna Supur1 and Damir Rebic2*
1Faculty of Medicine, University of Sarajevo, Bosnia and Herzegovina, Europe
2Clinic for nephrology, Clinical Center University of Sarajevo, Bosnia and Herzegovina, Europe
Damir Rebic, Clinic for nephrology, Clinical Center University of Sarajevo, Bosnia and Herzegovina, Europe
Received Date:December 13, 2023; Published Date:December 18, 2023
Abstract
Background: Monitoring of antimicrobial resistance is a key component of antibiotic stewardship programs. Cross sectional studies from of a
tertiary care center that have investigated favoring factors for fluoroquinolone-resistant Escherichia coli in man with urinary tract infection (UTI).
Methods: We performed a cross sectional study including 175 patients of the Department of Nephrology in whom E. coli was isolated from urine
or blood cultures in a two-year period. Clinical data were collected from patients’ records using a structured questionnaire. Multivariable analysis
was performed for the detection of risk factors.
Results: Fluoroquinolone -resistant E. coli was detected in 22% of patients. Risk factors for ciprofloxacin-resistant E. coli included prior use of fluoroquinolones (p<0.03), prior urinary tract catheterization (p<0.05) and recurrent UTIs (p<0.03). 60.8% of all prescriptions in urinary tract
infections were for fluoroquinolones, and this antibiotic class was the empiric antibiotic regimen of choice in 72.5% of all acute, uncomplicated,
urinary tract infections.
Conclusions: The increasing prevalence of fluoroquinolone-resistant E. coli makes empiric therapy in UTIs with this agent questionable,
especially in patients with one or several of the above-mentioned risk factors. Due to the increasing resistance rate, continuous surveillance and
susceptibility testing in individual patients, particularly with complicated UTIs, is indispensable for adequate therapy.
Keywords: Fluoroquinolone; resistance; urinary tract infection; escherichia coli
Abbreviations: UTI - Urinary Tract Infection; FQ s- Fluoroqinolone; E. coli - Escherichia coli; CLSI - Clinical and Laboratory Standards Institute
Introduction
Urinary tract infection (UTI) remains one of the most common clinical entities necessitating antimicrobial therapy. The emergence and spread of drug-resistant uropathogens, particularly Escherichia coli, even among community-acquired UTI, has limited treatment choices [1,2]. This is of particular concern in developing countries where the capacity for resistance surveillance and access to healthcare are limited and over-the-counter drug purchase is rampant in the community. Prevalence and risk factors for trimethoprim–sulfamethoxazole- resistant Escherichia coli among women with acute uncomplicated urinary tract infection in a developing country [3,4]. The degree to which these rates reflect prevalence in the community is unknown. Additionally, reported risk factors for resistance among truly community-acquired uropathogens are limited to settings in the developed world [5,6]. The dearth of such information is alarming in light of the emergence of highly drug-resistant com munity-associated strains in the region [7]. Across Europe, levels of antibiotic consumption show great variations, with the use of fluoroquinolones being highest in Portugal and Spain.
As the emergence of resistance is associated with high antibiotic consumption [8], it is not surprising that resistance to ciprofloxacin in E. coli shows great geographical variations, too, reaching high levels in southern Europe and low levels in northern European countries [9]. In addition to attentive monitoring of resistance patterns, the identification of risk factors for infections with resistant strains may contribute to improved empirical treatment. Factors associated with resistance to ciprofloxacin in E. coli reported in previous studies are urinary tract abnormalities, older age, previous antimicrobial therapy (especially quinolone therapy), urinary catheterization, recurrent UTIs, male gender and presence of complicated UTI [10]. Resistance rates of in- and outpatients at our hospital ranged from 0 to 40% in 2008 [unpublished internal report]. The aims of this study were to determine resistance rates in patients with UTIs, to assess prescribers’ choices for empirical antibiotic therapy, and to identify favoring factors for infections due to fluoroquinolone -resistant E. coli in patients at the Department of Nephrology, Sarajevo, Bosnia, and Herzegovina.
Methods
We performed a cross sectional study at the Department of Nephrology. All adult patients were included in whom E. coli was isolated from clinical urinary samples or blood cultures (in case of clinical symptoms suggestive of urinary tract infection but negative urinary culture) in the study period. Most of the patients presenting with complicated urinary tract infections are treated at the Department of Nephrology, including kidney transplant recipients. All specimens were tested in a central clinical microbiology laboratory in the Clinical Center. Bacteria were isolated from urine and blood cultures according to standard methods [11]. Antimicrobial susceptibility testing and screening for extended spectrum beta-lactamase (ESBL) was performed according to the Clinical and Laboratory Standards Institute (CLSI) [Clinical and Laboratory Standards Institute. 2008. Performance Standards for Antimicrobial Susceptibility Testing; Eigtheenth Informational Supplement 100-S18. Clinical and Laboratory Standards Instiute, Wayne, PA, USA.]. In the disk diffusion test, ciprofloxacin zone diameters of <15 mm and of >21 mm were considered resistant and susceptible, respectively; zones of 16–20 mm were considered as intermediately susceptible but were categorized as non-susceptible.
Some strains were tested by a commercial microdilution test, minimal inhibitory concentrations (MIC) of ciprofloxacin of <1 mg/L and of >4 mg/L were considered susceptible and resistant respectively; an MIC of 2 mg/L was intermediately susceptible but was categorized as non-susceptible. The screening test for ESBL was an inhibition zone of <22 mm against ceftazidime or of <27 mm against cefotaxime. Any synergy between amoxicillin/clavulanic acid and ceftazidime or cefepime (double disk method), or between piperacillin/tazobactam and cefotaxime in the disk diffusion test was also an indication for a confirmation test by Etest according to the prescription of the manufacturer (Biodisk, Sweden); a greater than twofold concentration decrease in an MIC for ceftazidime or cefepime, or for cefotaxime tested in combination with clavulanic acid versus its MIC when tested alone was confirmatory for ESBL. In accordance with the CLSI guidelines, all ESBL-producing E. coli strains were classified as resistant to all penicillin’s, cephalosporins and aztreonam regardless of the MICs determined for these drugs. Demographic and clinical information of both in- and outpatients was collected from each patient by chart review using a structured questionnaire.
Statistical analysis
Data were analysed using Stata Statistical Software, version 12.0 (StataCorp., College Station, TX, USA). Descriptive statistics were used to summarize patient characteristics and the prevalence of antimicrobial resistance. Univariate and multivariate analyses for putative risk factors for FQs-resistant E. coli were conducted. Logistic regression analysis was performed to identify risk factors for acquisition of fluoroquinolone-resistant E. coli. The level of significance was set at p < 0.05, using two-sided comparisons. The study was approved by the local Ethics committee.
Results
Of 229 patients with growth on culture, six had mixed pathogens. The most common organisms isolated were E. coli (76.2%), Staphylococcus saprophyticus (8.9%), and Klebsiella pneumoniae (3.4%) (Table 1). Four samples (2%) with ≥10 000 CFU/ml of coagulase- negative staphylococci as pure growth (n = 3) or mixed with S. saprophyticus (n = 1), with significant pyuria (>5/high-power field), were considered to have true pathogens. Patients’ characteristics, clinical presentation and microbiological findings are summarized in Table 2. The median age was 56.8 (51.9–60.4) years. The most common diagnosis was acute UTI (29%). FQs-resistant E. coli was isolated in 61 patients. 60.8% of initial antibiotic prescriptions were for fluoroquinolones, and 7% were for sulfamethoxazole-trimethoprim. Fluoroquinolones were the empiric antibiotic therapy of choice in 72.5% of all acute. The characteristics of patients with ciprofloxacin-resistant strains were compared with those of patients with a ciprofloxacin-susceptible strain (Table 3).
Table 1:Pathogens in urinary tract infections.
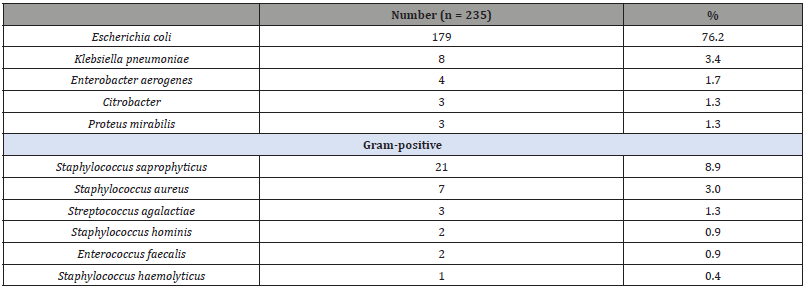
Table 2:Patients characteristics.

Table 3:Favoring factors for UTI with FQs-resistant E. coli.
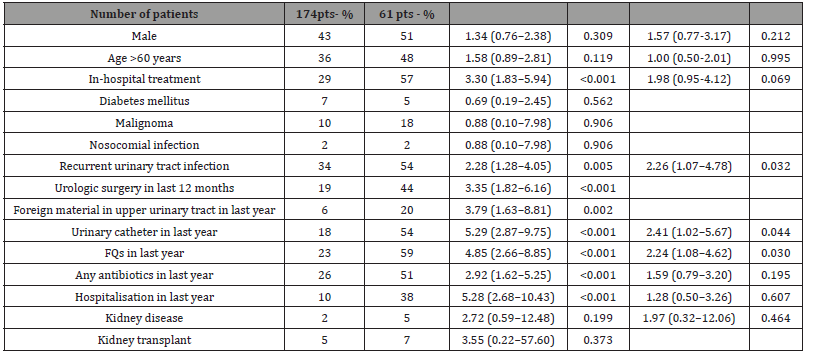
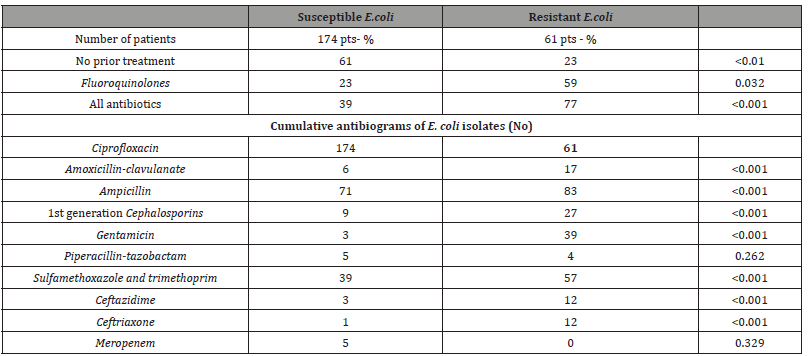
Significant predictors of ciprofloxacin-resistant E. coli in univariable analysis were hospital status, recurrent UTIs, urolithiasis, urinary catheter, prior use of fluoroquinolones, prior use of any antibiotics and prior treatment in the Department of Nephrology. To identify independent risk factors, a multivariable analysis was performed. Thereby, fluoroquinolone use in the preceding year (odds ratio [OR] (95% confidence intervals [CI]): 2.24 (1.08–4.62), p= 0.03), urinary tract catheterisation in the preceding year (OR: 2.41 (1.02–5.67), p= 0.04) and recurrent urinary tract infections (OR: 2.26 (1.07–4.78), p= 0.02) were found to be independently associated with infection or colonisation with a FQs-resistant strain. A further analysis of prior antibiotic use is depicted in Table 4. Only one cycle of fluoroquinolone treatment in the preceding year significantly increased the odds of being infected or colonised with a FQs-resistant E. coli strain, whereas one cycle of any antibiotic in last year did not. However, the odds were significantly increased when multiple antibiotic cycles were recorded, regardless of the antibiotic class used. The cumulative antibiograms of FQs-resistant and FQs-susceptible E. coli strains are shown in Table 4 too.
Table 4:Antibiotic treatmen in last year and cumulative antibiogram of E. coli isolates.
Discussion
This study provides the prevalence and risk factors for antimicrobial resistance of uropathogens among inpatients with UTI. Two other prospective studies from developing countries enrolled adult outpatients with community-acquired UTI but included both men and women with uncomplicated and complicated infections [12,13]. Thus, their findings, which as expected showed much higher rates of antimicrobial resistance, may not be comparable to ours. The aim of this study was to identify risk factors for colonization or infection with FQs-resistant E. coli in patients treated at the Department of Nephrology. Recurrent urinary tract infections, urinary catheterization within the last year and use of fluoroquinolones in the last year turned out to be independently associated with FQs-resistant strains. Those resistant strains were often also resistant to other antibiotics, mostly against ampicillin. Fluoroquinolones turned out to be the most frequently prescribed antibiotics for treatment of acute UTI, and pyelonephritis.
Our study documents risk factors for UTIs caused by FQs-resistant E. coli in patients at a tertiary care center. The population studied is exceptional in respect to the high prevalence of diseases that facilitate urinary tract infections, for example urinary tract obstructions, or foreign material in the upper and lower urinary tract. The study has several limitations. Charts of outpatients are usually not as detailed as those of inpatients, probably accounting for systematic deviations in the availability of information. In outpatients, antibiotic therapy for UTI is usually started empirically and cultures are only performed if the patient fails to respond to treatment, has recurrent episodes of UTI or has complicated UTI [14,15]. Thus, data on resistance rates based on laboratory surveillance may overestimate the true levels of antibiotic resistance in the community, accounting for selection bias. Among the potential risk factors assessed for FQs resistance, only the number of UTI episodes in the previous year was shown to be a significant predictor. Prior antimicrobial use (which has classically been linked to resistance), regardless of class and time elapsed before the incident episode, did not predict resistance.
This lack of association was unexpected and difficult to reconcile with the finding of the number of past UTI episodes (which would likely trigger antimicrobial intake) as a risk factor. Failure to find an association could be due to a type II error, i.e., the association could be in a range below what our study was able to detect. Data on time elapsed were not available for 19% of those who had claimed past antimicrobial exposure, thus decreasing the sample available for risk assessment. Moreover, we had to rely on individual recall of antimicrobial usage and could not quantify the degree of exposure in terms of regimen duration and dosage. Thus, we could have missed identifying any possible link between individual antimicrobial exposure and resistance. In a systematic review of the effect of antimicrobial prescribing in primary care on drug resistance in individual patients, a dose–response relationship, particularly for trimethoprim and amoxicillin, was observed [16]. Prior to this meta-analysis, several studies that attempted to assess the relationship between antimicrobial consumption and E. coli resistance showed conflicting results.
Risk factors for FQs-resistant E. coli in UTIs have been presented by other authors [17,18]. The results of our study are in agreement with risk factors previously reported, with prior exposure to fluoroquinolones being the most commonly described factor to increase risk. Whereas other authors tried to find an association with the presence of a urinary catheter at the time of detection of the ciprofloxacin-resistant strain [19], we included prior urinary tract catheterization within one year as a possible risk factor. However, multivariable analysis did not reveal prior urinary tract catheterization as an independent risk factor. Others have been able to show a correlation between age and fluoroquinolone resistance [20], whereas we could not. Differences between studies may have arisen due to different settings, different populations under study, and, to some extent, disregard of collinearity and co-causality in multivariable analyses.
During the study period, FQs was only recommended as a firstline agent in the treatment of uncomplicated acute pyelonephritis. The results of our study show that those recommendations were not followed properly, as FQs turned out to be the most frequently prescribed antibiotic for treatment of acute, uncomplicated UTIs. The fact that FQs are often given inappropriately is alarming. Some authors demonstrated that most participants underestimated fluoroquinolone use in the management of acute, uncomplicated lower UTI and that they considered the high level of usage to be inappropriate and creates risk of increased resistance [21]. This suggests that part of decreasing the usage of fluoroquinolones is to inform clinicians about their high prescription rate as they tend to underestimate their use and are sometimes not aware of the high selection pressure. How generalizable are our findings? In increasingly urbanized developing countries with rising incomes and ready access to healthcare, comparable antimicrobial resistance patterns may be observed in community pathogens causing acute uncomplicated UTI.
Unfortunately, this remains conjectural because similar prospective studies from other developing countries have yet to be reported. Several other factors such as non-human antimicrobial usage, the availability of substandard drugs, a lack of antimicrobial stewardship, and other environmental influences would need to be considered to determine the applicability of our findings to developing countries in the region.
Conclusion
In summary, our findings support the recommendation for the use of FQs, and not TMP–SMX, as the first-line empiric treatment for acute uncomplicated UTI. Based on the low resistance profile, oral first- and second-generation cephalosporins appear to be acceptable. Unfortunately, such antimicrobial choices are more costly than TMP–SMX. Nonetheless, resistance of E. coli is not only selected using fluoroquinolones, but also using unrelated antimicrobial classes including ampicillin, amoxicillin, trimethoprim, or sulfamethoxazole alone, and trimethoprimsulfamethoxazole [22,23]. Therefore, to decrease selection pressure to those ‘classic’ antibiotics, more frequent use of other antibiotics, for example nitrofurantoin and Fosfomycin, may be considered. In complicated UTIs, microbiological testing is essential to ensure adequate therapy because of high resistance rates. Our findings need to be confirmed by larger studies in more diverse settings and replicated in other developing countries.
References
- Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, et al. (2011) International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52(5): e103-e120.
- Hooton TM, Besser RR, Foxman BB, Fritsche TR, Nicolle LE (2004) Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin Infect Dis 39(1): 75-80.
- Louie MG, Marissa A, Henson KE, Ata RM, Lopez M, et.al. (2015) International surveillance reports of increasing resistance rates of community-acquired coli fluoroquinolones (FQs), and other frequently used drugs for the treatment of UTI are mostly derived from the laboratory- and hospital-based collection of urinary isolates.
- Kahlmeter G, Poulsen HO (2012) Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO·SENS study revisited. Int J Antimicrob Agents 39(1): 45-51.
- Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, et al. (2007) Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case–control study. J Antimicrob Chemother 60(1): 92-99.
- Filiatrault L, McKay RM, Patrick DM, Roscoe DL, Quan G, et al. (2012) Antibiotic resistance in isolates recovered from women with community-acquired urinary tract infections presenting to a tertiary care emergency department. CJEM 14(5): 295-305.
- Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, et al. (2005) Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis 5(8): 481-493.
- Goossens H, Ferech M, Vander Stichele R, Elseviers M (2005) Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365(9459): 579-587.
- Kahlmeter G (2003) An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother 51(1): 69-76.
- Arslan H, Azap OK, Ergonul O, Timurkaynak F (2005) Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother 56(5): 914-918.
- Clarddige JE, Johnson JR, Pezzlo M (1998) Laboratory diagnosis of urinary tract infections. Cumitech 3B. American Society for Microbiology, Washington, DC, USA.
- Goebel MC, Trautner BW, Grigoryan L (2021) The Five Ds of Outpatient Antibiotic Stewardship for Urinary Tract Infections. Clin Microbiol Rev 34(4): e0000320.
- Casey JA, Rudolph KE, Robinson SC, Bruxvoort K, Raphael E, et al. (2021) Sociodemographic Inequalities in Urinary Tract Infection in 2 Large California Health Systems. Open Forum Infect Dis 8(6): ofab276.
- Bilsen MP, Jongeneel RMH, Schneeberger C, Platteel TN, Nieuwkoop C, et al. (2023) Definitions of Urinary Tract Infection in Current Research: A Systematic Review. Open Forum Infect Dis 10(7): ofad332.
- Walker GK, Yustyniuk V, Shamoun J, Jacob ME, Correa M, et al. (2022) Detection of Escherichia coli and Enterococcus spp. in dogs with polymicrobial urinary tract infections: A 5-year retrospective study. J Vet Intern Med 36(4): 1322-1329.
- St-Jean A, Chateau D, Dahl M, Ernst P, Daneman N, et al. (2021) Regional variation in the potentially inappropriate first-line use of fluoroquinolones in Canada as a key to antibiotic stewardship? A drug utilization review study. BMC Infect Dis 21(1): 733.
- Tang YH, Lu PL, Huang HY, Lin YC (2022) Clinical effectiveness of beta-lactams versus fluoroquinolones as empirical therapy in patients with diabetes mellitus hospitalized for urinary tract infections: A retrospective cohort study. PLoS One 17(3): e0266416.
- Vermeulen H, Coenen S, Hens N, Bruyndonckx R (2021) Impact of changing reimbursement criteria on the use of fluoroquinolones in Belgium. J Antimicrob Chemother 76(10): 2725-2732.
- Shariati A, Arshadi M, Khosrojerdi MA, Abedinzadeh M, Ganjalishahi M, et al. (2022) The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front Public Health 10: 1025633.
- Adamus-Białek W, Wawszczak M, Arabski M, Majchrzak M, Gulba M, et al. (2019) Ciprofloxacin, amoxicillin, and aminoglycosides stimulate genetic and phenotypic changes in uropathogenic Escherichia coli Virulence 10(1): 260-276.
- Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G (2015) Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis 15: 545.
- Reis AC, Santos SR, Souza SC, Saldanha MG, Pitanga TN, et al. (2016) Ciprofloxacin Resistance Pattern among Bacteria Isolated from Patients with Community-Acquired Urinary Tract Infection. Rev Inst Med Trop Sao Paulo 58: 53.
- Sørum V, Øynes EL, Møller AS, Harms K, Samuelsen Ø, et al. (2022) Evolutionary Instability of Collateral Susceptibility Networks in Ciprofloxacin-Resistant Clinical Escherichia coli mBio 13(4): e0044122.
-
Velma Rebic, Mufida Aljicevic, Amela Dzubur, Edna Supur and Damir Rebic*. Favoring Factors for Urinary Tract Infections due to Fluoroquinolone-Resistant Escherichia Coli in A Tertiary Care Department – Single Center Experience. Annals of Urology & Nephrology. 3(5): 2023. AUN.MS.ID.000574.
-
Regulatory dendritic cells, Kidney transplantation, Cell therapy, Specific immune microenvironment, Immunosuppressive agents, Kidney immunology, Genetic modification, Human leukocyte, Genetic modification, Drug intervention, Cytokine exposure, Immunosuppressant, Renal parenchyma
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






